
04 Nov REMDESIVIR
THE EMERGENCY-APPROVED ANTIVIRAL THERAPY
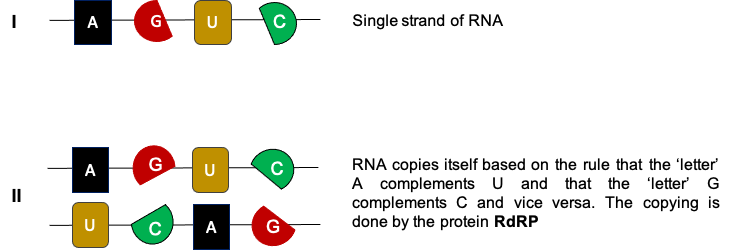
SARS-Cov-2, the novel coronavirus that causes COVID-19 disease, is a virus keeping all its genetic info in a single strand of RNA. RNA – the ‘cousin’ of DNA – is a polymeric molecule essential in many biological procedures with regards to the regulation of genes. DNA & RNA molecules are nucleic acids and along with lipids, proteins, and carbohydrates, they constitute one of the four major macromolecules essential for all known forms of life. Like DNA, RNA is assembled as a chain of nucleotides made of a combination of four different ‘letters’: A, U, G, C (instead of the letters A, T, G, C that make up DNA) (Figure 1). It is found in nature as a single strand (Figure 1), as opposed to the paired double strand of DNA molecules (found in human cells, for example).
In order for viruses to survive, they need to copy their genetic material inside a host. So, every time a virus wants to ‘copy’ itself (replicate in the scientific language), it has to make a copy of its RNA sequence/ chain of letters. Remdesivir drug prevents this process by giving the viral RNA copier (RdRP: RNA-dependent RNA Polymerase) ‘fake’ RNA letters to use (Figure 2). The drug seems to help some people at least to a certain extent, while no other medicine has such a reported effect so far. As a result, it got Emergency Use Authorization from the FDA in May 2020, meaning doctors can give it to hospitalized patients outside of clinical trials. So, how exactly does it work and why isn’t it exactly a miracle drug?
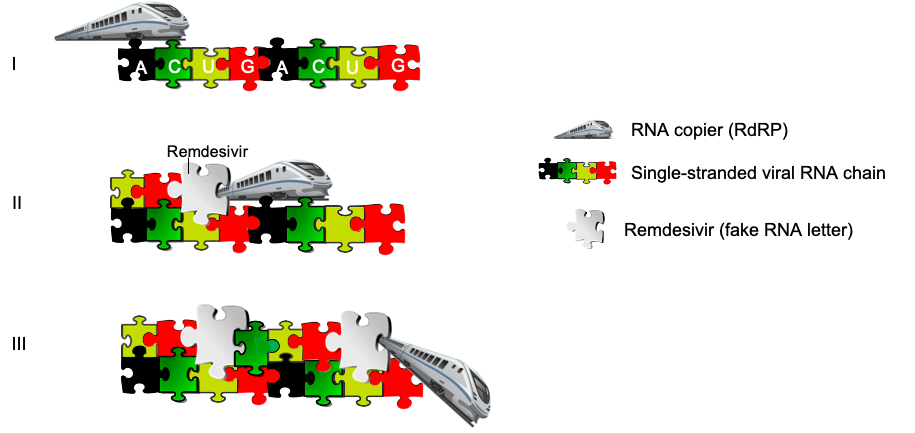
A simplified diagram of Remdesivir’s interference during RNA copying (replication). RNA copier (RdRP) — like a train — travels on a half-track (single-strand RNA of the virus) (I) and adds the pieces for the other half of the track ahead of it as it goes (II). Remdesivir ‘fools’ the copier and gets added as a normal RNA letter; the copier keeps adding a few more until it actually has to terminate the process as the new RNA chain synthesized has a weird structure which blocks the train (copier) and is not functional. As a result, the train (copier) ‘derails’ and the viral RNA has not copied itself successfully (III). Animation: https://www.thebioteacher.com. All rights reserved.
A common strategy for antivirals is Nucleoside/Nucleotide Analogs (NAs), in other words ‘fake’ RNA letters. But because SARS-CoV-2 has a proofreader, it is quite hard to trick it. There is a protein domain (ExoNuclease domain) able to remove fake letters, but the RNA copier (RdRP) has to first sense that there is a problem before it can act and remove the fake letters. This is where remdesivir could potentially ‘take control’. It is a trickster – it mimics the RNA letter adenosine (A) so well that the RNA copier (RdRP) adds it to the growing new RNA chain as it makes copies and does this so well that the virus’ proofreaders don’t cut the fake letters out, but not well enough that the virus can make functional RNA copies from it. The virus starts copying its RNA, adds remdesivir thinking it is a normal RNA letter, then adds a few more letters and finally the ‘copier’ (see train on Figure 2) gets stuck and has to terminate the replication – likely due to the extra parts of remdesivir (compared to normal A letters) clashing with its RNA copier (RdRP) as it gets threaded through the exit (Figure 2).
Different NAs have different Mechanisms of Action (MoAs). Remdesivir acts as a⠀
– Non-obligate chain terminator: This class of drugs lets the RNA copier (RdRP) add a couple of RNA letters during replication process (copying of itself), but then the polymerase has to stop because the messed up fake letters made the RNA structure strange (Figure 2).
Remdesivir, produced by Gilead pharmaceutical company, was initially found in a screen for hepatitis C virus (HCV). It was later provided to the Centers for Disease Control & prevention (CDC) and the U.S. Army Medical Research Institute of Infectious Diseases as part of a library of molecules the government agencies could screen for effectiveness against various infectious diseases. Scientists found it was potentially useful against Ebola, but it was put aside after another treatment for Ebola was found to work better (monoclonal antibodies). However, since Gilead had already taken it quite far in the testing process, scientists had data on its safety profile and its production process.
In a trial of 1,063 patients, remdesivir resulted in shortening the average hospitalization time from 15 to 11 days. Moreover, it lowered the death rate from a percentage of 11.9% recorded in the placebo group to 7.1% in the treated group. Although the result was not statistically significant, it was promising and it therefore led to the initial Emergency Use Authorization (EUA) in May 2020, which only authorized it for patients with severe COVID-19 symptoms (patients who needed oxygen, etc.). On August 28, the FDA broadened the scope of the EUA to allow its administration to anyone who is hospitalized and diagnosed with COVID-19, regardless of how severe their symptoms are.
Indeed, any substance that helps reduce hospital stays will relieve burden on hospitals. In that terms, remdesivir offers a (temporary at least) solution. However, it has not proved that helpful for very sick patients, which makes for a problematic aspect. Further trials of remdesivir are underway and results will be out soon to shed more light on how this type of antivirals could potentially mitigate SARS-CoV2 virus’ fast multiplication inside its host human cells and eventually make the patients benefit.
Another disadvantage of remdesivir is that it has to be given intravenously which makes delivering it to many problematic. On top, remdesivir prevents the virus from replicating (copying itself); it might therefore be much more effective early on, before the virus really starts to take hold. In other words, it might be more effective in patients who are pre-symptomatic or mildly symptomatic – and these patients are unlikely to be the ones in hospitals.
For making a more optimized antiviral, remdesivir could potentially serve as a starting point and preferably designed in a swallowable form. In science it is, however, often the case that such ‘cool ideas’ are practically difficult to follow for various biochemical reasons. Nevertheless, more scientific evidence is about to be published soon and this and only this will and can pave the way on all the hypotheses hanging around this celebrity antiviral drug.



Sorry, the comment form is closed at this time.