15 Nov IMMUNOLOGIC MEMORY
FOR HOW LONG ARE WE PROTECTED AFTER AN INFECTION OR A VACCINE?
One new vaccine against SARS-CoV-2 is out and while this is already considered a big breakthrough for the novel coronavirus pandemic, scientists around the globe remain conservatively optimistic about it.
There are three key points we should keep in mind according to the scientific community:
1. While the Phase 3 trial of Pfizer and BioNTech looked indeed very positive, more data are needed to enhance our knowledge about its safety and its potential for big scale production. (In January 2021 the first vaccinations have already taken place and we are therefore exactly in that phase of more data collection regarding responses and effectiveness).
2. It needs to be kept at -80°C, which makes for an important issue when it comes to its proper and efficient handling, as well as distribution.
3. Finally, only time along with more data would allow us to know better how well the vaccine will work and/or for how long.
So, scientists are reminding us that important safety measures, like regularly washing hands, wearing masks and keeping distances, are still key to combatting the virus.
Figure 1| The two main defense lines of our immune system; the non-specific (innate) response (shown on the left) and the specific (adaptive) response (shown on the right).
But what exactly do we mean by ‘how long’ the vaccine will work?
The longevity of a vaccine’s benefits has to do with the ‘immunologic memory’. Before we dive into ‘immunologic memory’ however, let’s first have a look on the body’s response to any kind of pathogen. Our body has two levels of protection; a non-specific and a specific one (Figure 2).
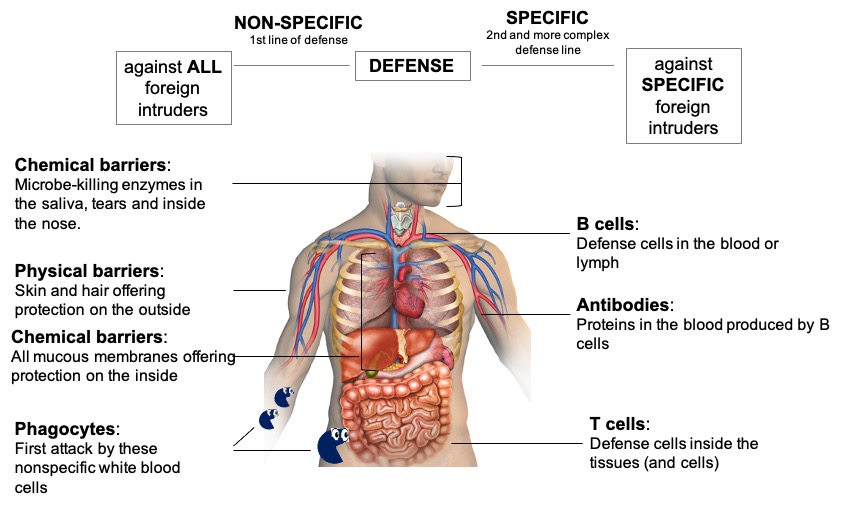
1. The very first response to the pathogens is a non-specific response. The body is constantly defending us against attacks from pathogens. The first line of defense against infection stops the pathogens from entering the body. These are general defenses and are not specific to certain types of pathogens; they can be physical or chemical barriers. This non-specific or innate response can also be further sub-divided to two levels.
The first sub-level comes with the most basic protection, meaning organs — like the skin and hair — which make up a physical barrier, as well as enzymes found in the saliva of our mouth, tears, and stomach acids, which act as chemical barriers against pathogens by breaking them down.
The second sub-level of the non-specific response are the phagocytes. Etymologically originating from the Greek word ‘phagein’, ‘φαγεῖν’, meaning ‘to eat’, phagocytes are cells which are literally ‘eating up’ pathogens when they encounter them. These white blood cells are attracted to pathogens either through chemicals secreted by the pathogens or through receptors on phagocytes’ membranes ‘recognizing’ the foreign intruders. The phagocytes surround bacteria and viruses in the blood, engulf them and break them down using enzymes. After breaking down the pathogens, they release chemical signals causing the attraction of more ‘fighters’ to the battlefield, a process known as, inflammation. Some of the phagocytes transfer a portion of the ‘chopped-up’ microbe on their surface and use it as a signal to elicit a more elaborate immune response later on. This chopped-up piece is called antigen and it is part of the microbe sufficient to cause an immune response.
2. But this system is not specific to viruses. It will not learn and it will not give you immunity if the virus re-enters your body in the future. This is a job which will be done by the second level of protection, called the specific or (adaptive) immune response. Contrary to the non-specific immune response, which exists across many species, the specific responseis thought to be a newer adaptation of evolution. The main actors here are the lymphocytes — another type of white blood cells (leukocytes)-.
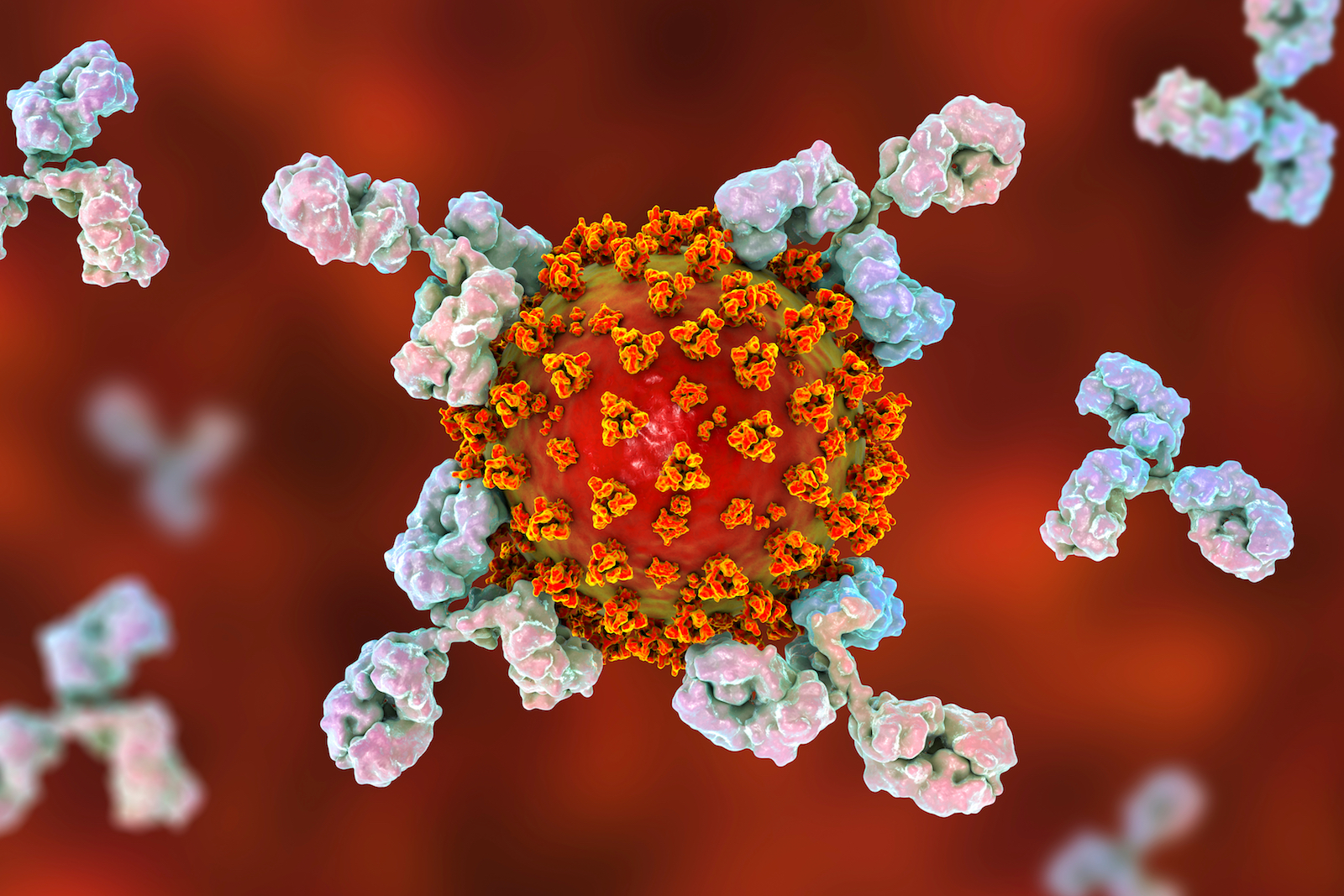
Lymphocytes can be divided to B and T lymphocytes (or simply B and T cells) (Figure 3). B cells are produced in the bone marrow and they are involved in what we call ‘humoral response’. These cells are the ones producing antibodies, which are -simply put- proteins that can ‘stick’ to the virus (and any kind of pathogen) and ‘neutralize’ it (Figure 1). They do that through a ‘key-lock recognizing’ relationship and this recognition occurs in the lymph or blood, and not inside the cell, thereby the name: humoral (‘humor’ is a medieval term for body fluid).
T cells, although originating also from the bone marrow, mature in the thymus. They can be further categorized to the helper-T-cells, which signal B cells to go make antibodies and the cytotoxic-T-cells, which are involved in what we call ‘cell-mediated response’. They can attack and kill infected cells, as well as communicate the propagation of more ‘fighters’ to the battlefield. Therefore, the cell-mediated immunity protects against intracellular (inside the cell) pathogens as opposed to the humoral immunity, which protects against extracellular (outside the cell) pathogens.
The non-specific and specific responses are bridged through one type of phagocytes (there are many types, e.g. neutrophils, which are fast and the most abundant ones, macrophages, etc.), the dendritic cells (Figure 3). The main function of these cells is to carry antigens (part of the pathogen) to peripheral lymphoid organs and present them to T lymphocytes. When a dendritic cell takes up a pathogen, it becomes activated and travels to a nearby lymph node. On activation, the dendritic cell matures into a highly effective antigen-presenting cell (APC) (Figure 3). Activated dendritic cells secrete chemical substances called cytokines that influence both the non-specific (innate) and the specific (adaptive) immune responses, making these cells essential ‘gatekeepers’.
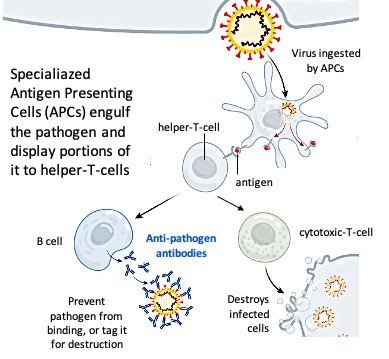
Memory and specificity: Two key characteristics of the adaptive immune response
The specific (adaptive) immune system accomplishes the task of defense against microbes at two levels: first by generating a specific immune response (namely antibodies) against the invading pathogen in order to control the infection; and secondly by remembering the pathogen in case this enters the body again in the future. Immune memory can take the form of a constant vigilance (circulating antibody) and/or the ability to mount an accelerated immune response upon re-exposure to the same pathogen (memory-T-cells, memory-B-cells). This rapid recall response can either completely prevent a disease, or greatly lessen the severity of clinical symptoms.
The first documentation of immune memory dates back to the time of the Greek historian Thucydides who recorded that the ‘same man was never attacked twice’ while describing the plague of Athens in 430 B.C. It is remarkable that nothing was known about the immune system or about microbes when Thucydides made his astute observations on immune memory; it would be more than 2000 years before we gained an appreciation of the immune system and learned that microbes cause infectious diseases.
But while the immune system ‘remembers’ some infections clearly and protects us for several years through antibodies, it has a habit of forgetting others, much like our own memory. Measles for example, is highly memorable; one infection is enough to offer life-long immunity. However, there are microbes, such as RSV (Respiratory Syncytial Virus), which can infect children multiple times within the same winter.
Since a vaccine is — in its core — the pathogen given to us (usually) in a weakened form in order to drive the body to generate antibodies against it without making us too sick, immunologic memory applies to vaccines too.
Researchers have been trying for years to figure out why some vaccines protect for mere weeks while others work for life. According to Plotkin, who began to research vaccines in 1957, we simply don’t know the rules of inducing long-lasting immunity. ‘Everything depends on immunologic memory, and we have not systematically measured it.’ ‘I keep saying, it’s not well understood, it’s not well understood’, says Bali Pulendran, an immunologist at Stanford University, California, who has reached the same conclusion about vaccine durability.
And while some vaccines drive the immune system to mount effective responses for decades, if not an entire human life, others have to be repeated after some years due to declining number of antibodies. The vaccine against the cancer-causing, sexually transmitted human papillomavirus (HPV), for example, has proven remarkably durable, while the vaccination for tetanus has to be repeated after ten years in order to boost the number of antibodies.
The new coronavirus, Sars-CoV-2, has not been around long enough to allow us know for sure how long immunity lasts after infection. Nonetheless, there are six other human coronaviruses that could give us an idea. Four of them have the symptoms of the common cold and immunity is short-lived. Studies have shown that some patients could be re-infected even within a year.
Research at King’s College London also suggested levels of antibodies that kill coronavirus waned over a three-month study. But even if antibodies disappear, the B cells that manufacture them, may still be around. B cells for the Spanish Flu, for example, have been found in people 90 years after that pandemic. If the same holds true for COVID-19, then a second infection would be milder than the first. It is also not understood what happens to T cells in the long term. Interestingly, T cells against the original Sars virus (Severe Acute Respiratory Syndrome) have been found 17 years later. Once again, we reach the conclusion, that we can’t know for sure yet. Science requires time and big data. Only time and the collection of a big number of data will allow us to reach solid, evidence-based conclusions.
Until then, we can wash our hands* and have common sense.
Disclaimer: Washing our hands is a -if not THE- key strategy to reduce possibility of catching and transmitting a microbe. It shall therefore always be followed regardless the presence or absence of effective drugs and vaccines (alongside other safety measures).



Sorry, the comment form is closed at this time.